Hampton Research蛋白结晶试剂盒






Products > Crystallization Screens > PEG/pH • PEG/pH 2 > PEG/pH • PEG/pH 2 • PEG/pH HT
PEG/pH • PEG/pH 2 • PEG/pH HT
Applications
- Crystallization screen where Polyethylene glycol 4,000 and buffers sample an acidic to neutral, and/or neutral to alkaline pH
Features
- Developed at Hampton Research
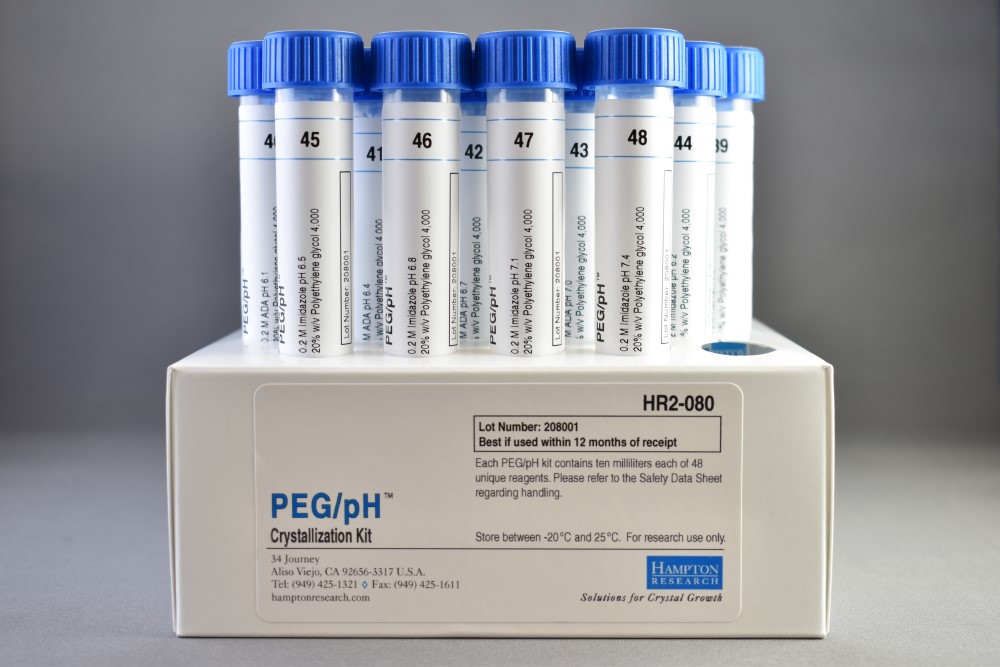
- PEG/pH samples pH 3.5 – 7.4 in 0.2 M buffer
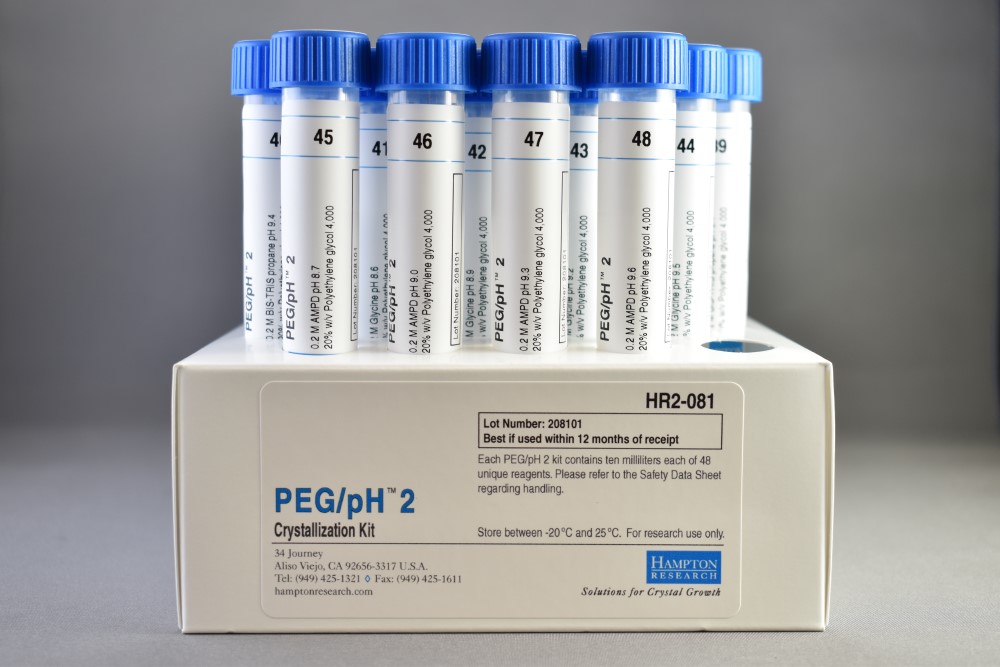
- PEG/pH 2 samples pH 6.4 – 9.6 in 0.2 M buffer
- PEG/pH HT samples pH 3.5 – 9.6 in 0.2 M buffer
- Polyethylene glycol 4,000 – primary reagent
- PEG/pH & PEG/pH 2 each utilize 10 unique buffers, 0.2 M – secondary reagent
- PEG/pH HT 20 unique buffers, 0.2 M – secondary reagent
- Compatible with vapor diffusion, microbatch, free interface diffusion
Description
pH is an effective solubility, stability and crystallization variable because most proteins demonstrate pH dependent solubility minima and will solubilize, precipitate, or crystallize at particular pH values or in the presence of specific buffers. The solubility minima may correspond with the isoelectric point (pI) of the protein, but this is not always the case. The solubility minima and maxima is often complex and may depend on other chemical and physical variables in the crystallization experiment.
Using PEG/pH, PEG/pH 2, or PEG/pH HT one isolates pH, buffer type and relative supersaturation from other chemical and physical variables to screen the effect that pH and buffer type have on the solubility, stability, homogeneity, monodispersity and crystallization of the sample. Varying the pH can alter the protonation state and charge of amino acid residues in the protein, generating different species of the protein for solubility and crystallization screening. The change in pH can have a dramatic effect on inter and intramolecular contacts in the protein and can manipulate how the protein interacts with itself, the surrounding solvent and chemicals in the drop. By screening buffer type and pH in a low ionic strength environment of Polyethylene glycol, PEG/pH simultaneously delivers as a solubility and a crystallization screen for proteins.
Designed as a 96 reagent screen, but offered as two sets of 48 x 10 ml reagents or a single 96 x 1 ml reagent block, PEG/pH, PEG/pH 2, and PEG/pH HT samples Polyethylene glycol 4,000 versus 20 different buffers, at 0.2 M, while sampling pH 3.5 – 9.6.
PEG/pH 1 samples 20% w/v Polyethylene glycol 4,000 versus pH 3.5 – 7.4 in 0.2 M buffer. PEG/pH is supplied in a 48 x 10 ml tube format. PEG/pH is compatible with vapor diffusion, free interface diffusion, and microbatch crystallization methods. For research use only.
PEG/pH 2 samples 20% w/v Polyethylene glycol 4,000 versus pH 6.4 – 9.6 in 0.2 M buffer. PEG/pH 2 is supplied in a 48 x 10 ml tube format. PEG/pH 2 is compatible with vapor diffusion, free interface diffusion, and microbatch crystallization methods. For research use only.
PEG/pH HT samples 20% w/v Polyethylene glycol 4,000 versus pH 3.5 – 9.6 in 0.2 M buffer. PEG/pH HT is supplied in a 96 x 1 ml block format. PEG/pH HT is compatible with vapor diffusion, free interface diffusion, and microbatch crystallization methods. For research use only.

Click to Zoom In
CAT NO
HR2-080
NAME
DESCRIPTION
10 ml, tube format
PRICE
$276.00
cart quote
CAT NO
HR2-081
NAME
DESCRIPTION
10 ml, tube format
PRICE
$276.00
cart quote
CAT NO
HR2-461
NAME
DESCRIPTION
1 ml, Deep Well block format
PRICE
$161.00
cart quote
Support Material(s)
 HR2-080 PEG/pH 1 Documents
HR2-080 PEG/pH 1 Documents HR2-080 PEG/pH 1 SDS
HR2-080 PEG/pH 1 SDS HR2-081 PEG/pH 2 Documents
HR2-081 PEG/pH 2 Documents HR2-081 PEG/pH 2 SDS
HR2-081 PEG/pH 2 SDS HR2-461 PEG/pH HT Documents
HR2-461 PEG/pH HT Documents HR2-461 PEG/pH HT SDS
HR2-461 PEG/pH HT SDS PEG/pH Formulation & Scoring Data
PEG/pH Formulation & Scoring Data References
1. Introduction to protein crystallization. Alexander McPherson and Jose A. Gavira. Acta Crystallographica Section F Volume 70, Issue 1, pages 2–20, January 2014.
2. Optimization of crystallization conditions for biological macromolecules. Alexander McPherson and Bob Cudney. Acta Crystallographica Section F Volume 70, Issue 11, pages 1445–1467, November 2014.
3. Protein Isoelectric Point as a Predictor for Increased Crystallization screening efficiency. Katherine A. Kantardjieff and Bernhard Rupp. Bioinformatics (2004) 20.
4. A protein crystallization strategy using automated grid searches on successively finer grid screens. Patricia C. Weber. Methods: A Companion to Methods in Enzymology. Vol. 1, No. 1, August, pp. 31-37, 1990.
5. Two approaches to the rapid screening of crystallization conditions. Alexander McPherson. Journal of Crystal Growth 122 (1992) 161-167.
6. Optimization of buffer solutions for protein crystallization. R. A. Gosavi, T. C. Mueser and C. A. Schall. Acta Cryst. (2008). D64, 506-514.
7. Buffer Solutions The Basics. R.J. Beynon and J.S. Easterby. 1996. IRL Press.


Hampton Research, first in crystallization since 1991, developing and delivering crystallization and optimization screens, reagents, plates, and other tools for the crystallization of biological macromolecules, including proteins (antibody), peptides (insulin), and nucleic acids (DNA).
- Products
- Gallery
- My Account
|
|
|
- Contact Us
- Quick Order
- Support
|
- Privacy Policy
- Terms and Conditions
|
- Products
- Gallery
- My Account
- Support
- Contact Us
- Quick Order
- Privacy Policy
- Terms and Conditions
|
|
|
|
|
|
|
© 2022 HAMPTON RESEARCH CORP.
| Website by Skyhound Internet