Hampton Research蛋白结晶试剂盒






Products > Crystallization Screens > SaltRx 1 • SaltRx 2 > SaltRx 1 • SaltRx 2 • SaltRx HT
SaltRx 1 • SaltRx 2 • SaltRx HT
Applications
- Primary or secondary, salt and pH matrix crystallization screen for biological macromolecules
Features
- Salt versus pH matrix crystallization screen
- Samples pH 4 to 9
- 22 unique salts versus salt concentration and pH
- Compatible with microbatch, vapor diffusion, liquid & gel diffusion methods
- Tube or Deep Well block format
- Preformulated, ready to screen
- Developed at Hampton Research
- All salts and buffers in screen readily available as Optimize™ reagents for reproducing and optimizing crystals
- Individual reagents available through the Hampton Research Custom Shop
Description
SaltRx was developed by Hampton Research as a primary and secondary, salt and pH based crystallization screen for biological macromolecules.
Salt is the only primary crystallization reagent (precipitant) utilized. Based on a design of 96 conditions, the screen evaluates a broad portfolio of crystallization salts of varying concentration and pH. The selection, concentration, and pH of the salts were determined by data mining the BMCD1 and other crystallization databases, crystallization reports in the literature, and internal crystallization trials performed at Hampton Research. Based on this analysis, up to 35% of protein crystallizations involve salt as the primary crystallization reagent. SaltRx can be used as a primary crystallization screen when salt, ionic strength and pH are desired or suspected as appropriate crystallization variables. It is also useful as a secondary screen when salt only reagents/conditions from screens such as Index™, Crystal Screen™, and Grid Screen™ produce crystals and when further screening for additional salt conditions or optimization is desired. Since this screen does not contain volatile organics, it is compatible with microbatch, vapor diffusion, liquid and gel diffusion crystallization methods. SaltRx may also be used for dialysis crystallization in conjunction with Dialysis Buttons™.
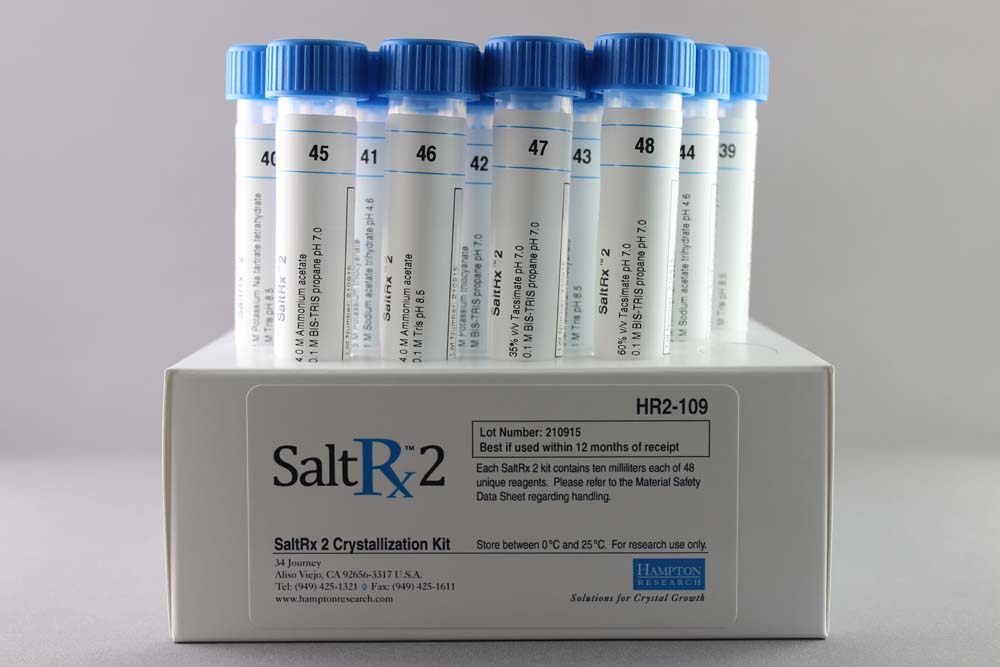
The original SaltRx kit (HR2-108) contained 96 unique reagents, 10 ml each. This kit has been discontinued and converted into two 48 reagent based kits, SaltRx 1 and SaltRx 2, available separately, which when used together are identical to the original SaltRx.
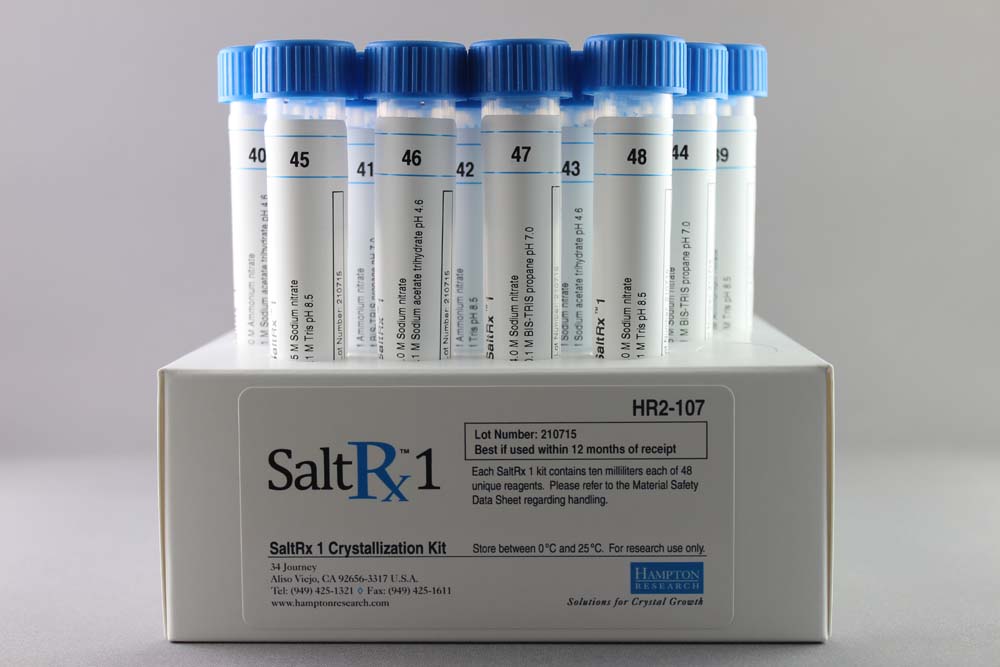
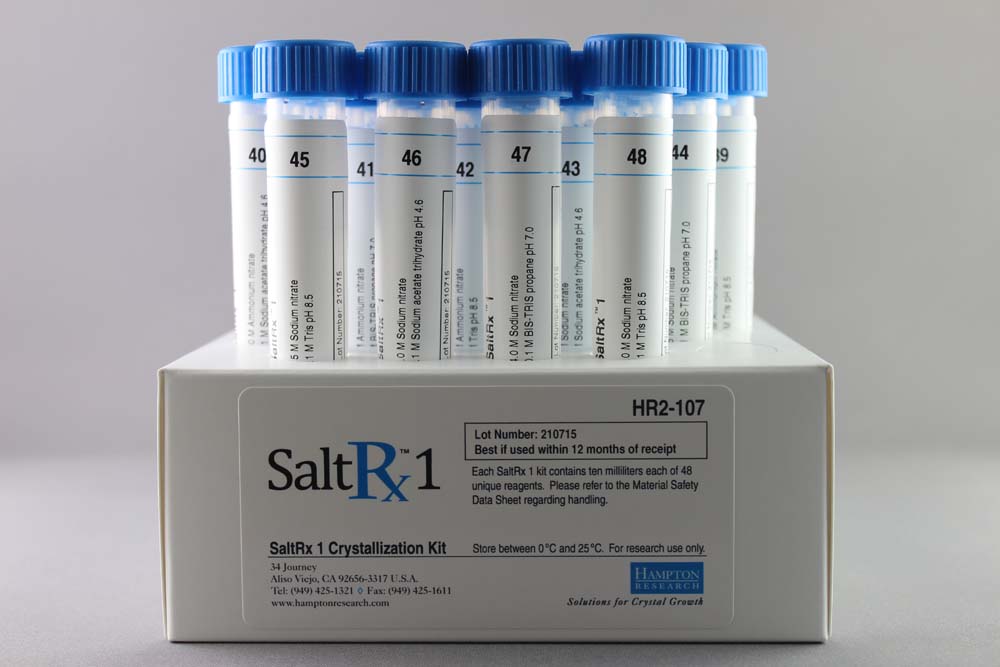
SaltRx 1 contains 48 unique reagents, 10 ml each. SaltRx 1 contains reagents 1-48 of the original SaltRx 96 reagent kit.
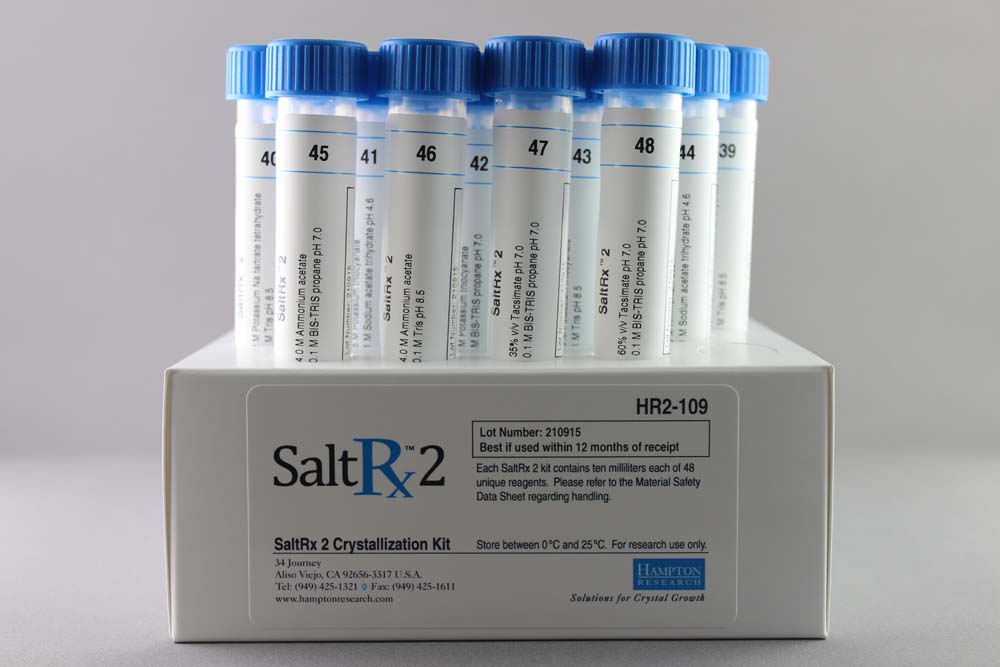
Salt Rx 2 contains 48 unique reagents, 10 ml each. SaltRx 2 contains reagent 49-96 of the original SaltRx 96 reagent kit.
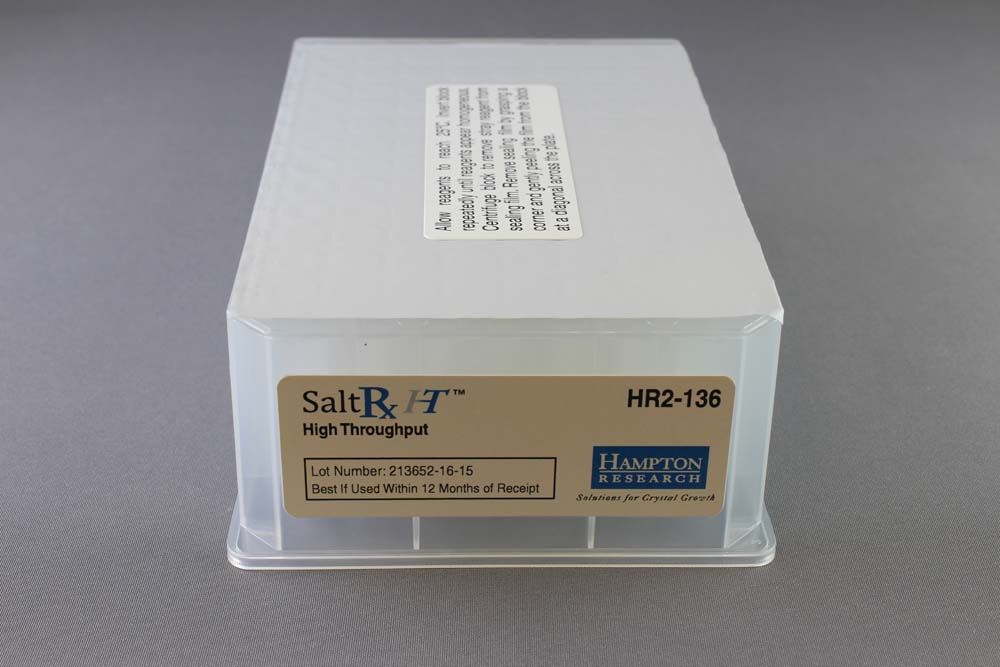
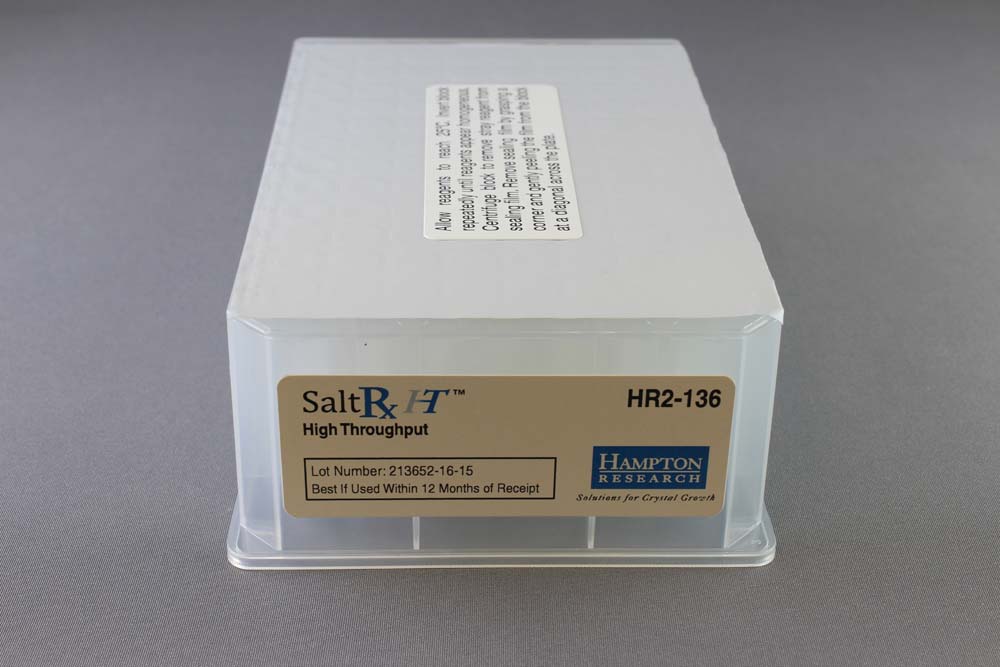
SaltRx HT contains the 96 SaltRx 1 and SaltRx 2 reagents in a single Deep Well block format.
Ready-to-use reagents are sterile filtered and formulated with ultra-pure Type 1 water, using the highest purity salts, polymers, organics and buffers. Individual reagents are available through the Hampton Research Custom Shop.
Measured pH range of kit is 4.1 to 9.0 at 25°C
Average measured pH of kit is 6.9 at 25°C
Median measured pH of kit is 7.3 at 25°C
Mode measured pH of kit is 7.4 at 25°C

Click to Zoom In
CAT NO
HR2-107
NAME
DESCRIPTION
10 ml, tube format
PRICE
$276.00
cart quote
CAT NO
HR2-109
NAME
DESCRIPTION
10 ml, tube format
PRICE
$276.00
cart quote
CAT NO
HR2-136
NAME
DESCRIPTION
1 ml, Deep Well block format
PRICE
$161.00
cart quote
Support Material(s)
 HR2-107 SaltRx 1 Documents
HR2-107 SaltRx 1 Documents HR2-107 SaltRx 1 SDS
HR2-107 SaltRx 1 SDS HR2-109 SaltRx 2 Documents
HR2-109 SaltRx 2 Documents HR2-109 SaltRx 2 SDS
HR2-109 SaltRx 2 SDS HR2-136 SaltRx HT Documents
HR2-136 SaltRx HT Documents HR2-136 SaltRx HT SDS
HR2-136 SaltRx HT SDS SaltRx Formulation & Scoring Data
SaltRx Formulation & Scoring Data Related Item(S)
- Individual SaltRx1 • SaltRx2 • SaltRx HT Reagents
References
1. Cloning, expression, purification and preliminary X-ray diffraction studies of a mycobacterial protein implicated in bacterial survival in macrophages. A. E. Shahine, P. Y. Chan, D. Littler, J. Vivian, R. Brammananth, P. K. Crellin, R. L. Coppel, J. Rossjohn and T. Beddoe. Acta Cryst. (2013). F69, 566-569.
2. Gilliland, G.L., Tung, M., Blakeslee, D.M. and Ladner, J. 1994. The Biological Macromolecule Crystallization Database, Version 3.0: New Features, Data, and the NASA Archive for Protein Crystal Growth Data. Acta Crystallogr. D50 408-413.
3. Crystallization and preliminary crystallographic analysis of molybdenum-cofactor biosynthesis protein C from Thermus thermophilus. S. P. Kanaujia, C. V. Ranjani, J. Jeyakanthan, S. Baba, L. Chen, Z.-J. Liu, B.-C. Wang, M. Nishida, A. Ebihara, A. Shinkai, S. Kuramitsu, Y. Shiro, K. Sekar and S. Yokoyama. Acta Cryst. (2007). F63, 27-29.
4. Crystal-contact engineering to obtain a crystal form of the Kelch domain of human Keap1 suitable for ligand-soaking experiments. Stefan Horer, Dirk Reinert, Katja Ostmann, Yvette Hoevels and Herbert Nar. Acta Cryst. (2013). F69, 592-596.


Hampton Research, first in crystallization since 1991, developing and delivering crystallization and optimization screens, reagents, plates, and other tools for the crystallization of biological macromolecules, including proteins (antibody), peptides (insulin), and nucleic acids (DNA).
- Products
- Gallery
- My Account
|
|
|
- Contact Us
- Quick Order
- Support
|
- Privacy Policy
- Terms and Conditions
|
- Products
- Gallery
- My Account
- Support
- Contact Us
- Quick Order
- Privacy Policy
- Terms and Conditions
|
|
|
|
|
|
|
© 2022 HAMPTON RESEARCH CORP.
| Website by Skyhound Internet