Hampton Research蛋白结晶试剂盒






Products > Crystallization Screens > Grid Screens > Grid Screen Sodium Malonate
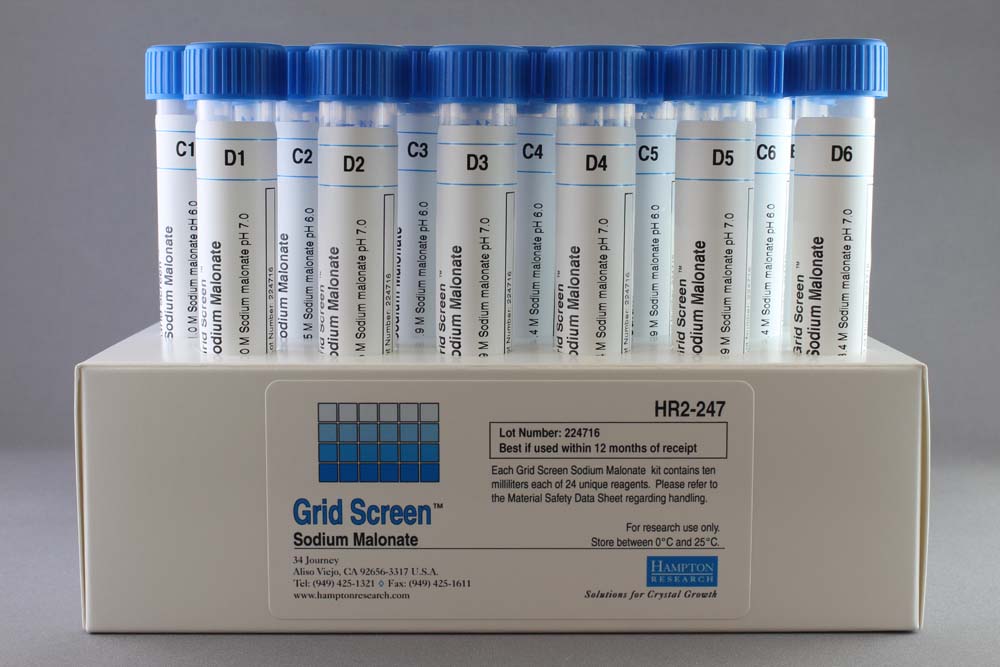
Grid Screen Sodium Malonate
Applications
- Primary or secondary, Sodium malonate versus pH grid crystallization screen for biological macromolecules
Features
- A systematic grid screen varying Sodium malonate concentration versus pH
- Samples pH 4 to 7
- Samples six concentrations of Sodium malonate
- Precise pH versus Sodium malonate screen
- pH adjusted after salt addition; 25°Celsius
- Combine reagents between rows or columns to create expanded grid screens
Description
Grid Screen™ Sodium Malonate is a preformulated reagent kit designed to provide a rapid screening method for the crystallization of biological macromolecules.
The screen is simple and practical for finding initial crystallization conditions as well as determining the solubility of a macromolecule in Sodium malonate between pH 4.0 and 9.0.
Grid Screen Sodium Malonate (HR2-247) evaluates Sodium malonate at six concentrations (1.0, 1.5, 1.9, 2.4, 2.9, 3.4 M) versus four pH levels (4, 5, 6, 7).
Grid Screen Sodium Malonate consists of 24 unique reagents, each supplied as 10 milliliter in sterile, screw cap tubes. Ready-to-use reagents are sterile filtered and formulated with ultra-pure Type 1 water, using the highest purity salt. Individual reagents are available through the Hampton Research Custom Shop.

Click to Zoom In
CAT NO
HR2-247
NAME
DESCRIPTION
10 ml, tube format
PRICE
$138.00
cart quote
Support Material(s)
 HR2-247 Grid Screen Sodium Malonate Documents
HR2-247 Grid Screen Sodium Malonate Documents HR2-247 Grid Screen Sodium Malonate SDS
HR2-247 Grid Screen Sodium Malonate SDS HR2-247 Grid Screen Sodium Malonte Formulation & Scoring Data
HR2-247 Grid Screen Sodium Malonte Formulation & Scoring Data Related Item(S)
- Individual Grid Screen Sodium malonate Reagents
References
1. Advance in Protein Chemistry Volume 41. Pages 1-33 (Patricia C. Weber). Academic Press, 1991.
2. Crystallization of nucleic acids and proteins, Edited by A. Ducruix and R. Giege, The Practical Approach Series, Oxford Univ. Press, 1992.
3. Current approaches to macromolecular crystallization. McPherson, A. Eur. J. Biochem. 189, 1-23, 1990.
4. Protein and Nucleic Acid Crystallization. Methods, A Companion to Methods in Enzymology, Academic Press, Volume 1, Number 1, August 1990.
5. A protein crystallization strategy using automated grid searches on successively finer grids. Patricia C. Weber. Methods: A Companion to Methods in Enzymology Vol. 1, No. 1, August, pp. 31-37, 1990.
6. Analysis of the human cofilin 1 structure reveals conformational changes required for actin binding. M. Klejnot, M. Gabrielsen, J. Cameron, A. Mleczak, S. K. Talapatra, F. Kozielski, A. Pannifer and M. F. Olson. Acta Cryst. (2013). D69, 1780-1788.
7. A comparison of salts for the crystallization of macromolecules. Alexander McPherson. Protein Science (2001), 10:418-422.


Hampton Research, first in crystallization since 1991, developing and delivering crystallization and optimization screens, reagents, plates, and other tools for the crystallization of biological macromolecules, including proteins (antibody), peptides (insulin), and nucleic acids (DNA).
- Products
- Gallery
- My Account
|
|
|
- Contact Us
- Quick Order
- Support
|
- Privacy Policy
- Terms and Conditions
|
- Products
- Gallery
- My Account
- Support
- Contact Us
- Quick Order
- Privacy Policy
- Terms and Conditions
|
|
|
|
|
|
|
© 2022 HAMPTON RESEARCH CORP.
| Website by Skyhound Internet