Hampton Research蛋白结晶试剂盒






Products > Optimization Screens > Solubility & Stability Screen 2 > Solubility & Stability Screen 2
Solubility & Stability Screen 2
Applications
- Solubility & Stability Screen 2 is designed to assist in the identification of optimal buffer, pH and ionic strength formulations that promote protein solubility and stability
- Sample buffer optimization for crystallization, cryo-electron microscopy, and NMR
Features
- Compatible with ThermoFluor (Differential Scanning Fluorimetry, DSF, Protein Thermal Shift) and Dynamic Light Scattering assays
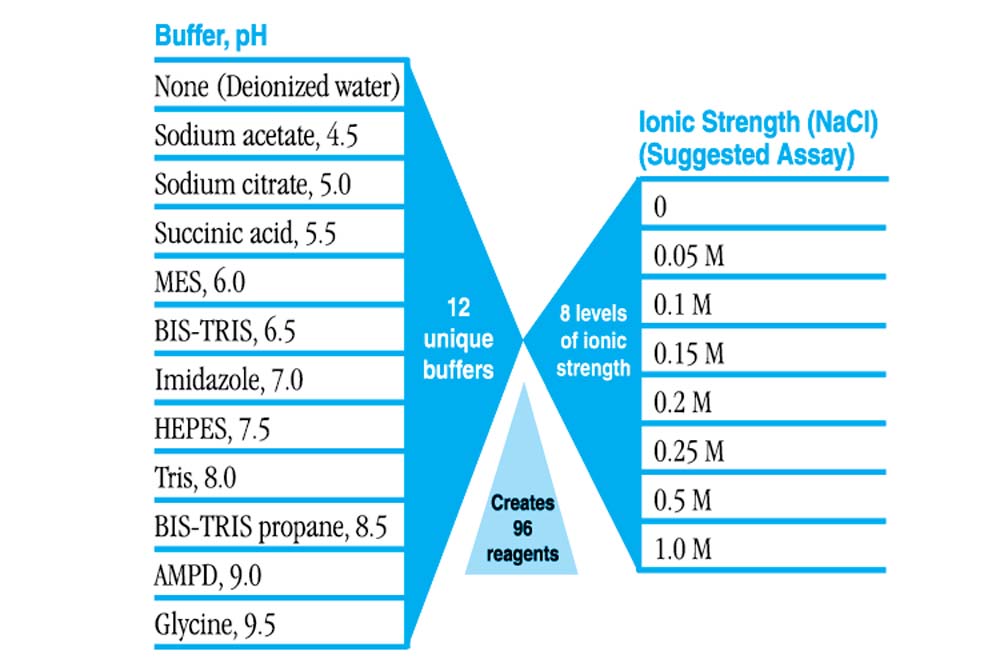
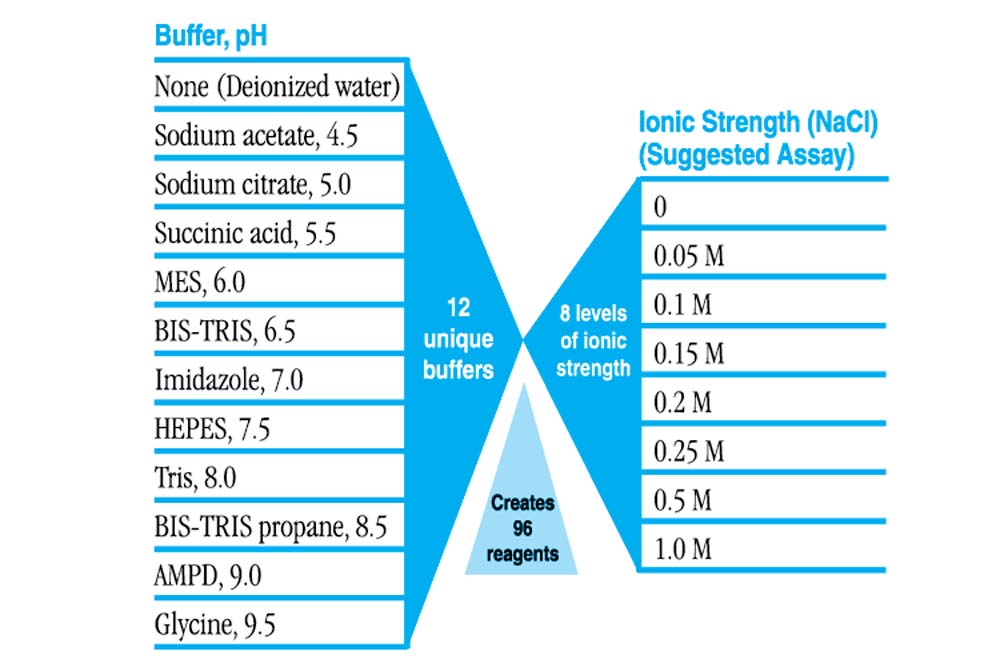
- Formulation of 12 unique buffers, pH 4.5 – 9.5, versus 8 levels of ionic strength
- Evaluate varying ionic strength in the presence and absence of buffer
- Evaluate varying buffer type and pH in different levels of ionic strength
- 96 sterile filtered reagents
- Concentrated reagent formulation
- Developed at Hampton Research
Description
Protein solubility and stability are universally required in a wide range of applications, including general biochemical studies, the preparation of proteins in diagnostics and pharmaceuticals, structural biology and crystallization.
The preparation of a concentrated, soluble and stable protein sample can often be a difficult task as proteins often aggregate, precipitate or denature.
Protein solubility and stability is affected by many different chemical factors including pH, buffer type, ionic strength and protein specific additives. pH, buffer type and ionic strength are dominant protein solubility and stability variables that can be evaluated and optimized using the Solubility and Stability Screen 2.
No clear correlation between intrinsic properties of proteins and solubility and stability exist, so systematic screening can help to identify optimal sample buffer conditions. Protein solubility and stability screening, performed using Dynamic Light Scattering and ThermoFluor assays together with the Solubility and Stability Screens and Slice pH can be an extremely data rich, informative, efficient, and cost-effective method for the identification of sample buffer conditions that maximally stabilize a protein for protein purification, formulation, crystallization, and functional characterization.
Dynamic Light Scattering (DLS) is an established technique for determining the size, monodispersity and polydispersity of proteins in solution. DLS with a plate reader allows a multitude of buffers, pH, ionic strength, protein specific additives and temperature to be screened. Combining Solubility & Stability Screens with DLS, particle size and size distribution, unfolding of proteins, crystallizability, thermal stability, aggregation and solubility behavior and be evaluated in a high throughput format.
The ThermoFluor assay is an established technique for assessing protein thermostability in solution. ThermoFluor allows systematic assessment of many reagents simultaneously, uses only small a small amount of protein, and is accessible to anyone with a real-time PCR instrument.
Once an optimal buffer, pH and ionic strength is identified, the sample can be exchanged into the new optimal buffer. Using this optimized sample, further ThermoFluor assays with the Solubility & Stability Screen, Silver Bullets, Silver Bullets Bio and Additive Screen can be performed to identify protein specific additives that further promote solubility and stability of the protein.
ThermoFluor® is a registered trademark of Johnson & Johnson.
Protein Thermal Shift is a trademark of ThermoFisher.
Sypro® is a registered trademark of ThermoFisher.

Click to Zoom In
CAT NO
HR2-413
NAME
DESCRIPTION
0.5 ml, Deep Well block format
PRICE
$301.00
cart quote
Support Material(s)
 HR2-413 Solubility & Stability Screen 2 Documents
HR2-413 Solubility & Stability Screen 2 Documents HR2-413 Solubility & Stability Screen 2 SDS
HR2-413 Solubility & Stability Screen 2 SDS Solubility & Stability Screen 2 Formulation & Scoring Data
Solubility & Stability Screen 2 Formulation & Scoring Data Related Item(S)
- Individual Solubility & Stability 2 Reagents
References
1. A thermal stability assay can help to estimate the crystallization likelihood of biological samples. Marquez et al. Acta Cryst. (2011). D67, 915-919.
2. The combined use of the ThermoFluor assay and ThermoQ analytical software for the determination of protein stability and buffer optimization as an aid in protein crystallization. Kevin Phillips and Andres Hernandez de la Pena. Current Protocols in Molecular Biology 10.28.1-10.28.15 April 2011.
3. Thermofluor-based optimization strategy for the stabilization and crystallization of Campylobacter jejuni desulforubrerythrin. Romao et al. Protein Expression and Purification 81 (2012) 193-200.
4. Optimization of protein solubility and stability for protein nuclear magnetic resonance. Bagby S, Tong KI, Ikura, M. Methods Enzymol. 2001;339:20-41.
5. High-Density Miniaturized Thermal Shift Assays as a General Strategy for Drug Discovery. Pantoliano et al, Journal of Biomolecular Screening, Volume 6, Number 6, 2001.
6. Thermofluor-based high-throughput stability optimization of proteins for structural studies. Ericsson, U.B. et al, Anal Biochem. 2006 Oct 15;357(2):289-98. Epub 2006 Aug 10.
7. Comparison of fluorescence and light scattering based methods to assess formation and stability of protein-protein complexes. J. Kopec, G. Schneider. Journal of Structural Biology 175 (2011) 216-223.
8. Enhancing recombinant protein quality and yield by protein stability profiling. Mezzasalma TM, Kranz JK, Chan W, Struble GT, Schalk-Hihi C, Deckman IC, Springer BA, Todd MJ. J Biomol Screen. 2007 Apr;12(3):418-28.
9. Optimization of protein purification and characterization using ThermoFluor screens. Boivin S, Kozak S, Meijers R. Protein Expr Purif. 2013 Oct;91(2):192-206. doi: 10.1016/j.pep.2013.08.002. Epub 2013 Aug 12.
10. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Frank H Niesen, Helena Berglund & Masoud Vedadi. Nat Protoc. 2007;2(9):2212-21.
11. Use of dynamic light scattering to assess crystallizability of macromolecules and macromolecular assemblies. Ferre-D′Amare AR1, Burley SK. Structure. 1994 May 15;2(5):357-9.
12. Crystallizing proteins – a rational approach? D′Arcy A. Acta Crystallogr D Biol Crystallogr. 1994 Jul 1;50(Pt 4):469-71.
13. Protein Crystallization. Techniques, Strategies, and Tips. A Laboratory Manual. Edited by Terese M. Bergfors. International University Line. 1999; 27-38.


Hampton Research, first in crystallization since 1991, developing and delivering crystallization and optimization screens, reagents, plates, and other tools for the crystallization of biological macromolecules, including proteins (antibody), peptides (insulin), and nucleic acids (DNA).
- Products
- Gallery
- My Account
|
|
|
- Contact Us
- Quick Order
- Support
|
- Privacy Policy
- Terms and Conditions
|
- Products
- Gallery
- My Account
- Support
- Contact Us
- Quick Order
- Privacy Policy
- Terms and Conditions
|
|
|
|
|
|
|
© 2022 HAMPTON RESEARCH CORP.
| Website by Skyhound Internet