| 产品编号 | 产品名称 | 产品规格 | 产品等级 | 产品价格 |
| 193-16711 | SuperSep Phos-tag™ (50 μmol/L), 10%, 13 well Phos-tag 13孔10%预制胶 |
5块 | – | – |
| 190-16721 | SuperSep Phos-tag™ (50 μmol/L), 10%, 17 well Phos-tag 17孔10%预制胶 |
5块 | – | – |
| 195-16391 | SuperSep Phos-tag™ (50 μmol/L), 12.5%, 13 well Phos-tag 13孔12.5%预制胶 |
5块 | – | – |
| 193-16571 | SuperSep Phos-tag™ (50 μmol/L), 12.5%, 17 well Phos-tag 17孔12.5%预制胶 |
5块 | – | – |
| 193-16691 | SuperSep Phos-tag™ (50 μmol/L), 15%, 13 well Phos-tag 13孔15%预制胶 |
5块 | – | – |
| 196-16701 | SuperSep Phos-tag™ (50 μmol/L), 15%, 17 well Phos-tag 17孔15%预制胶 |
5块 | – | – |
| 197-16851 | SuperSep Phos-tag™ (50 μmol/L), 17.5%, 13 well Phos-tag 13孔17.5%预制胶 |
5块 | – | – |
| 194-16861 | SuperSep Phos-tag™ (50 μmol/L), 17.5%, 17 well Phos-tag 17孔17.5%预制胶 |
5块 | – | – |
蛋白磷酸化研究的预制胶
SuperSep Phos-tag™
SuperSep Phos-tag™ 是研究蛋白磷酸化的方法,无需特异性磷酸化抗体或者同位素标记。
◆SuperSep Phos-tag™

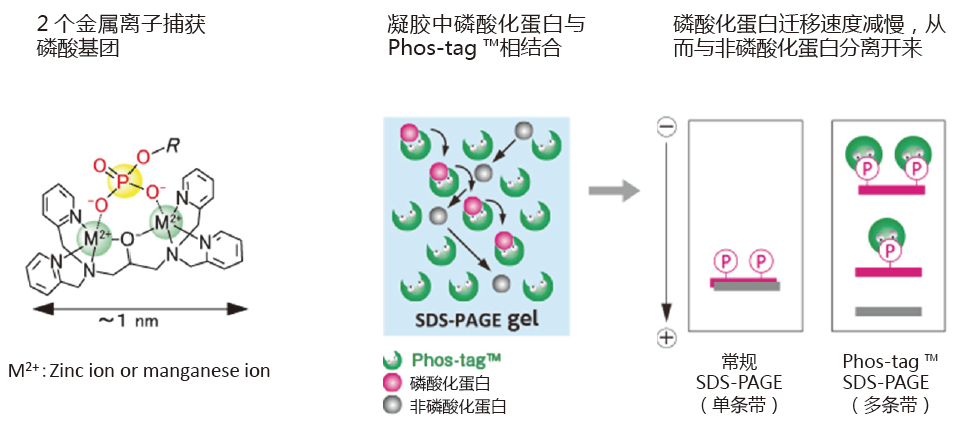
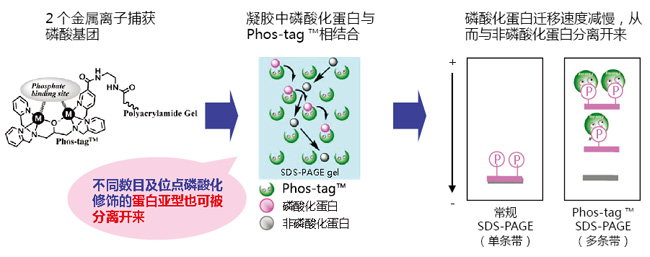
Phos-tag™ 是一种预制胶,预先加入了50 μmol/L的Phostag™ Acrylamide,打开包装即可直接使用。预制胶中含有锌作为金属离子,在中心凝胶缓冲液中保存稳定性很好,得到的带条结果也很整齐。
磷酸化蛋白和非磷酸化蛋白作为不同条带分离。
分离后,胶可用于考马斯亮蓝染色,免疫印迹和质谱实验。
已公开的验证蛋白列表,请点击
◆Phos-tag™ SDS-PAGE 的原理
◆在HighWire Search 上搜索到的论文数

◆运用
利用p35的丙氨酸突变体确定Cdk5 激活p35的磷酸化位点
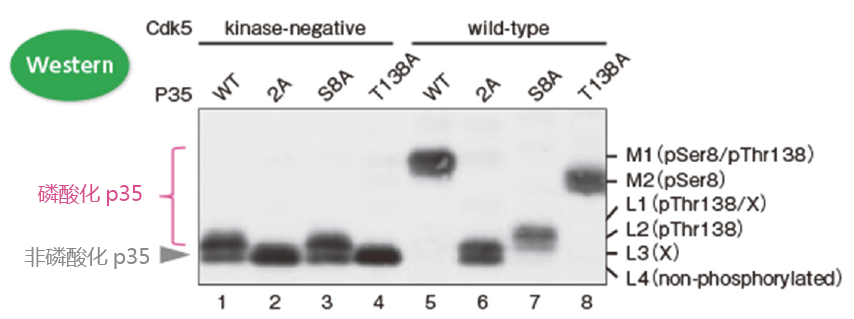
p35常见的磷酸化位点是Ser8和Thr138。但是Ser8和Thr138位点往往会发生丙氨酸突变,产生3种突变体(Ser8突变体:S8A,Thr138突变体:T138A,Ser8和Thr138双突变体:2A)。这3种突变体、野生型p35、Cdk5和没有激酶活性的Cdk5都来源于COS-7细胞。这些细胞裂解液用Phos-tag™ SDS-PAGE和Western blotting 进行检测(检测抗体:p35抗体)。
100 μM Phos-tag ™ 丙烯酰胺, 7.5% 聚丙烯酰胺凝胶
可明确磷酸化位点和条带迁移率的关系!
– 泳道1(条带L2和L4)和泳道5(条带M1):p35在Cdk5的作用下发生了磷酸化;
– 泳道1(条带L2和L4)和泳道3(条带L2和L4):在无激酶活性Cdk5的作用下,大约有一半p35蛋白在Thr138位点
发生磷酸化,同样在138位发生突变的p35蛋白亦是如此。
– 泳道5 (条带M1)和泳道6(条带L3和L4):Ser8和Thr138是主要的磷酸化位点;
– 泳道5(条带M1)、泳道7(条带L1和L2)和泳道8(条带M2):条带M1是Ser8和Thr138都发生磷酸化的条带;
条带M2是只有Ser8磷酸化的条带;条带L1和L2是只有Thr138磷酸化的条带。
※ 条带L1和L3中的X 是不确定哪个位点发生磷酸化的条带;
※ 条带L4是非磷酸化的p35。
【参考文献】
▪ Quantitative Measurement of in Vivo Phosphorylation States of Cdk5 Activator p35 by Phos-tag ™ SDS-PAGE. T. Hosokawa, T. Saito, A. Asada, K. Fukunaga, and S. Hisanaga,Mol. Cell. Proteomics, Jun 2010;9: 1133 – 1143.
【结果提供】
理化学研究所 脑科学综合研究中心 回路功能研究核心 记忆功能研究团队 细川智永(Dr. T. Hosokawa)
首都大学东京 理工学研究科 生命科学专业 神经分子功能研究室 久永真市(Dr. S. Hisanaga)
检测含有Dnmt1磷酸化激酶的片段
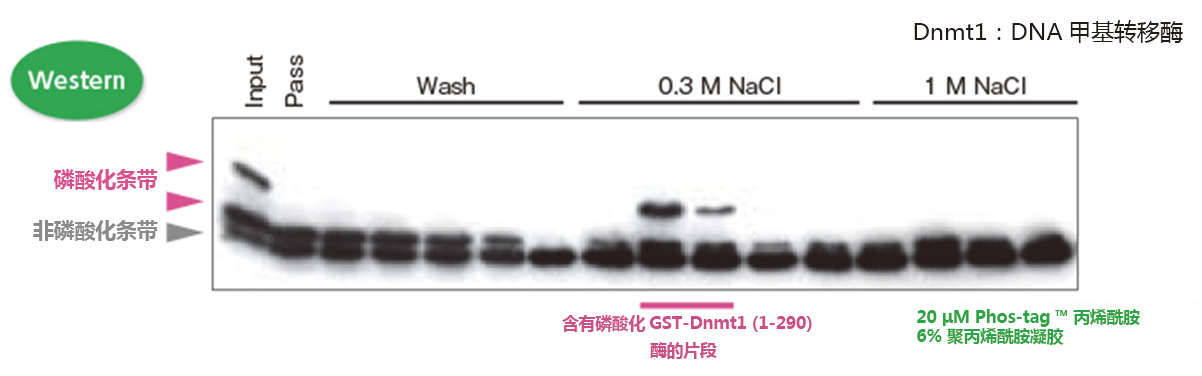
我们可以确定在片段中含有目的激酶!
① 采用亲和色谱法从鼠脑提取液中纯化GST-Dnmt1(1-290)结合蛋白
② 使用0.3 M 和1 M NaCl 的DNA 纤维素柱洗脱得到目的蛋白
③ GST-Dnmt1(1-290)作为体外激酶实验的反应底物
④ Phos-tag ™ SDS-PAGE 用于Western blotting,确定迁移条带中每个片段的激酶活性
【参考文献】
▪ The DNA-binding activity of mouse DNA methyltransferase 1 is regulated by phosphorylation with casein kinase 1delta/epsilon. Y. Sugiyama, N. Hatano, N. Sueyoshi, I. Suetake, S. Tajima, E. Kinoshita, E. Kinoshita-Kikuta, T. Koike, and I. Kameshita, Biochem. J.,
【结果提供】
高知大学 综合研究中心 生命、功能物质部门 实验实习机器设施 杉山康宪(Dr. Y. Sugiyama)
香川大学 农学部 应用生物科学科 动物功能生化学研究室 龟下勇(Dr. I. Kameshita)
二维电泳中的应用:分析hnRNP K磷酸化异构体
小鼠巨噬细胞J774.1 经LPS 刺激后,裂解细胞,经过免疫沉淀法分离得到hnRNP K。在二维电泳中,一维是IPG 胶,二维是Phos-tag ™ SDS-PAGE,可分离hnRNP K 的异构体。利用质谱仪,可以确认不同的点代表不同的亚型或修饰蛋白。
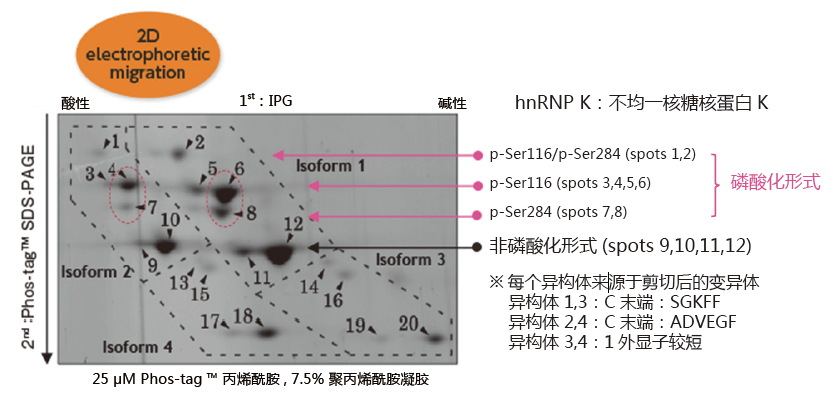
同一个等电点的位置上,不同位点发生磷酸化都可以被区分开来
(例: spots 6 vs. 8 and spots 4 vs. 7)
【参考文献】
▪ Characterization of multiple alternative forms of heterogeneous nuclear ribonucleoprotein K by phosphate-affinity electrophoresis. Y. Kimura, K. Nagata, N Suzuki, R. Yokoyama, Y. Yamanaka, H. Kitamura, H. Hirano, and O. Ohara, Proteomics, Nov 2010; 10(21): 3884-95.
【结果提供】
横滨市立大学 生命纳米系统科学研究科 生物体超分子系统科学专业 木村弥生(Dr. Y. Kimura)、平野久(Dr. H. Hirano)
理化学研究所RCAI 小原收
◆备注
样品制备:
Phos-tag SDS-PAGE对于蛋白样品中的杂质非常敏感,尤其是螯合剂,钒酸,无机盐,表面活性剂这类物质。
强烈建议在Phos-tag SDS-PAGE之前通过TCA沉淀或渗析法降低杂质含量。
转膜前处理:
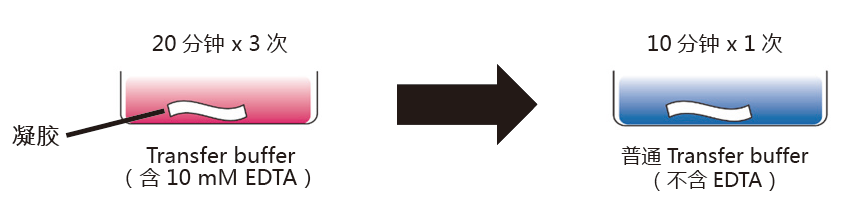
另一个必须的步骤是在转膜前,用EDTA去除凝胶中的金属离子(Mn2+或者Zn2+);
该步骤可提高蛋白的转膜效率。
● 分别准备10 mmol/L 含EDTA和不含EDTA 两种1x transfer buffer。
● 将凝胶浸泡在含10 mmol/L EDTA的1x transfer buffer,至少20分钟,温和摇晃。更换新缓冲液,重复3次。
● 将凝胶浸泡在不含10 mmol/L EDTA的1x transfer buffer,10分钟,温和摇晃。
● 转膜操作*。
* 建议用湿法转膜,以提高转膜效率。
◆质量控制
每一批SuperSep Phos-tag™,出厂前均根据其产品规格进行测试,以保证可分离磷酸化和非磷酸化蛋白,以及他们的分离成都在正常参数内。
◆保存温度
2-10℃
◆产品信息
用于Bio-Rad伯乐电泳仪
|
货号 |
品名 |
电泳仪 |
规格 |
|
198-17981 |
SuperSep™ Phos-tag™ (50μmol/L), 7.5%, 17well, 83×100×3.9mm |
Mini-PROTEAN® Tetra Cell (Bio-Rad Laboratories, Inc.) |
5 块 |
|
195-17991 |
SuperSep™ Phos-tag™ (50μmol/L), 12.5%, 17well, 83×100×3.9mm |
5 块 |
用于Life Technologies电泳仪
|
货号 |
品名 |
电泳仪 |
规格 |
|
192-18001 |
SuperSep™ Phos-tag™ (50μmol/L), 7.5%, 17well, 100×100×6.6mm |
XCell SureLock® Mini-Cell (Life Technologies, Inc.) |
5 块 |
|
199-18011 |
SuperSep™ Phos-tag™ (50μmol/L), 12.5%, 17well, 100×100×6.6mm |
5 块 |
用于Wako EasySeperator电泳仪
| 产品编号 | 产品 | 凝胶浓度 | 孔数 | 包装 |
| 192-17401 | SuperSep™ Phos-tag™ (50 μmol/L) Phos-tag™ 预制胶50 μmol/L |
6.0% | 13孔 | 5块 |
| 199-17391 | 6.0% | 17孔 | 5块 | |
| 195-17371 | 7.5% | 13孔 | 5块 | |
| 192-17381 | 7.5% | 17孔 | 5块 | |
| 193-16711 | 10.0% | 13孔 | 5块 | |
| 190-16721 | 10.0% | 17孔 | 5块 | |
| 195-16391 | 12.5% | 13孔 | 5块 | |
| 193-16571 | 12.5% | 17孔 | 5块 | |
| 193-16691 | 15.0% | 13孔 | 5块 | |
| 196-16701 | 15.0% | 17孔 | 5块 | |
| 197-16851 | 17.5% | 13孔 | 5块 | |
| 194-16861 | 17.5% | 17孔 | 5块 |
◆相关产品
| 产品编号 | 产品名称 | 规格 |
| 058-07681 | EasySeparator Phos-tag预制凝胶的配套电泳槽 |
1 set |
Phos-tag™ 系列
磷酸化蛋白新方法!
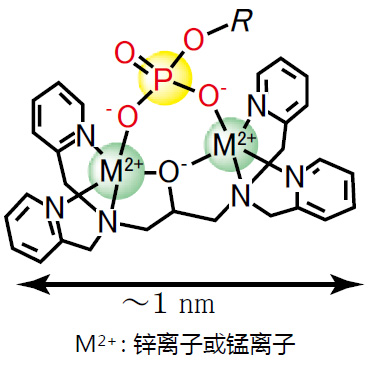
Phos-tag™是一种能与磷酸离子特异性结合的功能性分子。它可用于磷酸化蛋白的分离(Phos-tag™ Acrylamide)、Western Blot检测(Phos-tag™ Biotin)、蛋白纯化 (Phos-tag™Agarose)及质谱分析MALDI-TOF/MS (Phos-tag™ Mass Analytical Kit)。
◆Phos-tag™ 的基本结构:
特点:
与-2价磷酸根离子的亲和性和选择性高于其它阴离子
在pH 5-8的生理环境下生成稳定的复合物
◆原理:
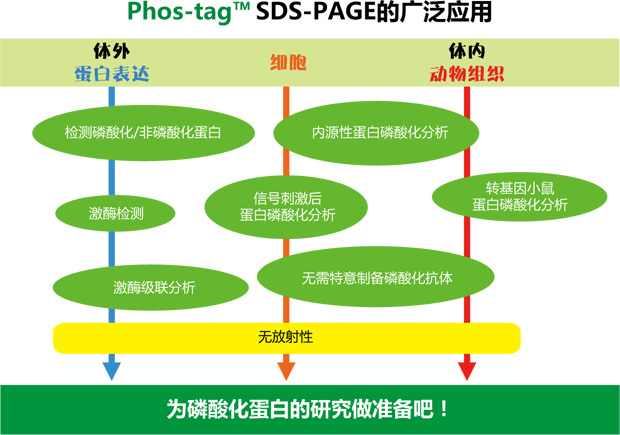
◆相关应用:
◆相关产品:
|
产品名称 |
用 途 |
|
Phos-tag™ Acrylamide |
分离: SDS – PAGE 分离不同磷酸化水平的蛋白 |
|
SuperSep Phos-tag™ |
分离: 预制胶中含有50μM Phos-tag™ Acrylamide |
|
Phos-tag™ Biotin |
检测: 代替 Western Blot 检测中的磷酸化抗体 |
|
Phos-tag™ Agarose |
纯化: 通用柱层析,纯化磷酸化蛋白 |
|
Phos-tag™ Mass Analytical Kit |
分析: 用于质谱 MALDI-TOF/MS 分析,提高磷酸化分子的检测灵敏度 |
phos-tag™由日本广岛大学研究生院医齿药学综合研究科医药分子功能科学研究室开发。
更多产品信息,请点击:http://phos-tag.jp

Phos-tag 第5版说明书

Phos-tag系列 ver 5
|
Q1. |
我们可以采用哪种凝胶染色法? |
|
A1. |
所有的染色法都可使用,最常用的例如考马斯亮蓝染色法,负染,银染和荧光染色等。 |
|
Q2. |
用考马斯亮蓝染法染色,着色不明显。 |
|
A2. |
在微波炉内进行脱色,会取得比较满意的效果。 方法:将染色的胶放在100毫升去离子水里,用擦拭纸包裹胶,再放进微波炉加热几分钟后更换去离子水,并重复上述步骤3或4次。请注意防止盛放胶的容器温度过高。 |
|
Q3. |
此款产品能否用于免疫印迹? |
|
A3. |
可以,如果用EDTA清除胶里面含有的锌,可以提高转膜的效率。 方法:胶浸在含有10 mmol/L EDTA的转移缓冲液(25 mmol/L tris、192 mmol/L甘氨酸,10%甲醇)里轻轻搅拌10分钟。重复上述步骤3次。然后放进不含EDTA的转移缓冲液(25 mmol/L tris、192 mmol/L的甘氨酸,10%甲醇)里搅拌10分钟并转移到PVDF膜或NC膜(硝酸纤维素膜)上。 |
|
Q4. |
条带扭曲了。 |
|
A4. |
含有EDTA,无机盐,表面活性剂等的样品可能会导致条带弯曲或者拖尾。通过TCA或透析沉淀脱盐样品。空白泳道也会导致相邻样品条带弯曲,在空白泳道加与样品相同体积的样品缓冲液(x1)。 |
|
Q5. |
磷酸化蛋白和非磷酸化蛋白不能分离。 |
|
A5. |
将β–酪蛋白(038-23221)作为Phos-tag SDS-PAGE电泳的阳性对照,将用碱性磷酸酶处理的β–酪蛋白作为阴性对照,并检查条带的迁移。如果只有一个条带,可能是由于Phos-tag™或丙烯酰胺的浓度没有优化导致磷酸化和非磷酸化蛋白不能分离。 |
|
Q6. |
可用在细胞的粗提物上吗? |
|
A6. |
可以的,可能Rf值可能有稍低,由于目的蛋白的因素,条带可能会比较模糊。 |
|
Q7. |
上样量多少? |
|
A7. |
1至5微克纯化的蛋白(考马斯亮蓝染法),10至30微克的组织或细胞提取物(取决于蛋白质的表达量)。 *这是推荐的用量,可以先进行常规的SDS-PAGE和免疫印迹法,确定合适的上样量。 |
|
Q8. |
使用哪种蛋白marker? |
|
A8. |
不推荐使用蛋白marker。该款产品不需要蛋白marker。因此,建议用来源于大肠杆菌的重组蛋白或者是非磷酸化样品作为阴性对照代替蛋白marker。 |
|
Q9. |
此款产品的配离子是什么? |
|
A9. |
锌离子 |
|
Q10. |
我们怎么知道是因为蛋白发生磷酸化条带才会发生迁移? |
|
A10. |
使用12.5% SuperSep™ Ace(Wako 目录No. 199-14971)进行电泳(有相同的胶浓度),检查目标蛋白质是否降解。 |
【参考文献】
· Conversion of graded phosphorylation into switch-like nuclear translocation via autoregulatory mechanisms in ERK signalling[J].Nature communications, 2016, 7,Shindo Y, Iwamoto K, Mouri K, et al.
· PTEN modulates EGFR late endocytic trafficking and degradation by dephosphorylating Rab7[J]. Nature communications, 2016, 7,Shinde S R, Maddika S.
· Feedback control of ErbB2 via ERK-mediated phosphorylation of a conserved threonine in the juxtamembrane domain[J]. Scientific Reports, 2016, 6: 31502,Kawasaki Y, Sakimura A, Park C M, et al.
· Plastid-nucleus communication involves calcium-modulated MAPK signalling[J]. Nature Communications, 2016, 7,Guo H, Feng P, Chi W, et al.
· Sequential domain assembly of ribosomal protein S3 drives 40S subunit maturation[J]. Nature communications, 2016, 7,Mitterer V, Murat G, Réty S, et al.
· Phos-tag analysis of Rab10 phosphorylation by LRRK2: a powerful assay for assessing kinase function and inhibitors[J]. Biochemical Journal, 2016: BCJ20160557,Ito G, Katsemonova K, Tonelli F, et al.
· Analysis of phosphorylation of the myosin targeting subunit of smooth muscle myosin light chain phosphatase by Phos-tag SDS-PAGE[J]. The FASEB Journal, 2016, 30(1 Supplement): 1209.1-1209.1,Walsh M P, MacDonald J A, Sutherland C.
· Using Phos-Tag in Western Blotting Analysis to Evaluate Protein Phosphorylation[J]. Kidney Research: Experimental Protocols, 2016: 267-277,Horinouchi T, Terada K, Higashi T, et al.
· The Abundance of Nonphosphorylated Tau in Mouse and Human Tauopathy Brains Revealed by the Use of Phos-Tag Method[J]. The American journal of pathology, 2016, 186(2): 398-409,Kimura T, Hatsuta H, Masuda-Suzukake M, et al.
· Phos-tag SDS-PAGE resolves agonist-and isoform-specific activation patterns for PKD2 and PKD3 in cardiomyocytes and cardiac fibroblasts[J]. Journal of Molecular and Cellular Cardiology, 2016,Qiu W, Steinberg S F.
· Analysis of phosphorylation of the myosin-targeting subunit of myosin light chain phosphatase by Phos-tag SDS-PAGE[J]. American Journal of Physiology-Cell Physiology, 2016, 310(8): C681-C691,Sutherland C, MacDonald J A, Walsh M P.
· Electrochemical biosensor for protein kinase A activity assay based on gold nanoparticles-carbon nanospheres, phos-tag-biotin and β-galactosidase[J]. Biosensors and Bioelectronics, 2016, 86: 508-515,Zhou Y, Yin H, Li X, et al.
· Validation of Cis and Trans Modes in Multistep Phosphotransfer Signaling of Bacterial Tripartite Sensor Kinases by Using Phos-Tag SDS-PAGE[J]. PloS one, 2016, 11(2): e0148294,Kinoshita-Kikuta E, Kinoshita E, Eguchi Y, et al.
· Phosphopeptide Detection with Biotin-Labeled Phos-tag[J]. Phospho-Proteomics: Methods and Protocols, 2016: 17-29,Kinoshita-Kikuta E, Kinoshita E, Koike T.
· A Phos‐tag SDS‐PAGE method that effectively uses phosphoproteomic data for profiling the phosphorylation dynamics of MEK1[J]. Proteomics, 2016,Kinoshita E, Kinoshita‐Kikuta E, Kubota Y, et al.
· Difference gel electrophoresis of phosphoproteome: U.S. Patent Application 15/004,339[P]. 2016-1-22,Tao W A, Wang L.
· ERK1/2-induced phosphorylation of R-Ras GTPases stimulates their oncogenic potential[J]. Oncogene, 2016,Frémin C, Guégan J P, Plutoni C, et al.
· Microtubules Inhibit E-Cadherin Adhesive Activity by Maintaining Phosphorylated p120-Catenin in a Colon Carcinoma Cell Model[J]. PloS one, 2016, 11(2): e0148574,Maiden S L, Petrova Y I, Gumbiner B M.
· Serine 231 and 257 of Agamous-like 15 are phosphorylated in floral receptacles[J]. Plant Signaling & Behavior, 2016, 11(7): e1199314,Patharkar O R, Macken T A, Walker J C.
· A small molecule pyrazolo [3, 4-d] pyrimidinone inhibitor of zipper-interacting protein kinase suppresses calcium sensitization of vascular smooth muscle[J]. Molecular pharmacology, 2016, 89(1): 105-117,MacDonald J A, Sutherland C, Carlson D A, et al.
· The RNA polymerase II C-terminal domain phosphatase-like protein FIERY2/CPL1 interacts with eIF4AIII and is essential for nonsense-mediated mRNA decay in Arabidopsis[J]. The Plant Cell, 2016: TPC2015-00771-RA,Chen T, Qin T, Ding F, et al.
· Vasorelaxant Effect of 5′-Methylthioadenosine Obtained from Candida utilis Yeast Extract through the Suppression of Intracellular Ca2+ Concentration in Isolated Rat Aorta[J]. Journal of agricultural and food chemistry, 2016, 64(17): 3362-3370,Kumrungsee T, Akiyama S, Saiki T, et al.
· Inhibition of deubiquitinating activity of USP14 decreases tyrosine hydroxylase phosphorylated at Ser19 in PC12D cells[J]. Biochemical and biophysical research communications, 2016, 472(4): 598-602,Nakashima A, Ohnuma S, Kodani Y, et al.
· Actin Tyrosine-53-Phosphorylation in Neuronal Maturation and Synaptic Plasticity[J]. The Journal of Neuroscience, 2016, 36(19): 5299-5313,Bertling E, Englund J, Minkeviciene R, et al.
· AMPK-dependent phosphorylation of lipid droplet protein PLIN2 triggers its degradation by CMA[J]. Autophagy, 2016, 12(2): 432-438,Kaushik S, Cuervo A M.
· Myocardin-related transcription factor a and yes-associated protein exert dual control in G protein-coupled receptor-and RhoA-mediated transcriptional regulation and cell proliferation[J]. Molecular and cellular biology, 2016, 36(1): 39-49,Olivia M Y, Miyamoto S, Brown J H.
· Extensive phosphorylation of AMPA receptors in neurons[J]. Proceedings of the National Academy of Sciences, 2016, 113(33): E4920-E4927,Diering G H, Heo S, Hussain N K, et al.
· The transmembrane region of guard cell SLAC1 channels perceives CO2 signals via an ABA-independent pathway in Arabidopsis[J]. The Plant Cell, 2016, 28(2): 557-567,Yamamoto Y, Negi J, Wang C, et al.
· The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP[J]. Journal of molecular and cellular cardiology, 2016, 90: 1-10,Kimura T E, Duggirala A, Smith M C, et al.
· Atg13 is essential for autophagy and cardiac development in mice[J]. Molecular and cellular biology, 2016, 36(4): 585-595,Kaizuka T, Mizushima N.
· The ChrSA and HrrSA two-component systems are required for transcriptional regulation of the hemA promoter in Corynebacterium diphtheriae[J]. Journal of Bacteriology, 2016: JB. 00339-16,Burgos J M, Schmitt M P.
· Intergenic Variable-Number Tandem-Repeat Polymorphism Upstream of rocA Alters Toxin Production and Enhances Virulence in Streptococcus pyogenes[J]. Infection and Immunity, 2016, 84(7): 2086-2093,Zhu L, Olsen R J, Horstmann N, et al.
· Receptor for advanced glycation end products (RAGE) knockout reduces fetal dysmorphogenesis in murine diabetic pregnancy[J]. Reproductive Toxicology, 2016, 62: 62-70,Ejdesjö A, Brings S, Fleming T, et al.
· Aurora kinase-induced phosphorylation excludes transcription factor RUNX from the chromatin to facilitate proper mitotic progression[J]. Proceedings of the National Academy of Sciences, 2016, 113(23): 6490-6495,Chuang L S H, Khor J M, Lai S K, et al.
· Quantitative phosphoproteomics of protein kinase SnRK1 regulated protein phosphorylation in Arabidopsis under submergence[J]. Journal of experimental botany, 2016: erw107,Cho H Y, Wen T N, Wang Y T, et al.
· Temporal regulation of lipin activity diverged to account for differences in mitotic programs[J]. Current Biology, 2016, 26(2): 237-243,Makarova M, Gu Y, Chen J S, et al.
· Block of CDK1‐dependent polyadenosine elongation of Cyclin B mRNA in metaphase‐i‐arrested starfish oocytes is released by intracellular pH elevation upon spawning[J]. Molecular reproduction and development, 2016, 83(1): 79-87,Ochi H, Aoto S, Tachibana K, et al.
· Mitotic Exit Function of Polo-like Kinase Cdc5 Is Dependent on Sequential Activation by Cdk1[J]. Cell reports, 2016, 15(9): 2050-2062,Rodriguez-Rodriguez J A, Moyano Y, Játiva S, et al.
· PLK2 phosphorylates and inhibits enriched TAp73 in human osteosarcoma cells[J]. Cancer medicine, 2016, 5(1): 74-87,Hu Z B, Liao X H, Xu Z Y, et al.
· Phosphorylated TDP-43 becomes resistant to cleavage by calpain: A regulatory role for phosphorylation in TDP-43 pathology of ALS/FTLD[J]. Neuroscience research, 2016, 107: 63-69,Yamashita T, Teramoto S, Kwak S.
· The Pch2 AAA+ ATPase promotes phosphorylation of the Hop1 meiotic checkpoint adaptor in response to synaptonemal complex defects[J]. Nucleic acids research, 2016: gkw506,Herruzo E, Ontoso D, González-Arranz S, et al.
· An optimized guanidination method for large‐scale proteomic studies[J]. Proteomics, 2016,Ye J, Zhang Y, Huang L, et al.
· Expression and purification of the kinase domain of PINK1 in Pichia pastoris[J]. Protein Expression and Purification, 2016,Wu D, Qu L, Fu Y, et al.
· BRI2 and BRI3 are functionally distinct phosphoproteins[J]. Cellular signalling, 2016, 28(1): 130-144,Martins F, Rebelo S, Santos M, et al.
· Identification of glycoproteins associated with HIV latently infected cells using quantitative glycoproteomics[J]. Proteomics, 2016,Yang W, Jackson B, Zhang H.
· Regulation of Beclin 1 Protein Phosphorylation and Autophagy by Protein Phosphatase 2A (PP2A) and Death-associated Protein Kinase 3 (DAPK3)[J]. Journal of Biological Chemistry, 2016, 291(20): 10858-10866,Fujiwara N, Usui T, Ohama T, et al.
· Regulatory Implications of Structural Changes in Tyr201 of the Oxygen Sensor Protein FixL[J]. Biochemistry, 2016, 55(29): 4027-4035,Yamawaki T, Ishikawa H, Mizuno M, et al.
· Histone demethylase Jmjd3 regulates osteoblast apoptosis through targeting anti-apoptotic protein Bcl-2 and pro-apoptotic protein Bim[J]. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2016, 1863(4): 650-659,Yang D, Okamura H, Teramachi J, et al.
· Analysis of Molecular Species Profiles of Ceramide-1-phosphate and Sphingomyelin Using MALDI-TOF Mass Spectrometry[J]. Lipids, 2016, 51(2): 263-270,Yamashita R, Tabata Y, Iga E, et al.
· Highly sensitive myosin phosphorylation analysis in the renal afferent arteriole[J]. Journal of Smooth Muscle Research, 2016, 52(0): 45-55,Takeya K.
· Functional dissection of the CroRS two-component system required for resistance to cell wall stressors in Enterococcus faecalis[J]. Journal of bacteriology, 2016, 198(8): 1326-1336,Kellogg S L, Kristich C J.
· Regulation of mitogen-activated protein kinase by protein kinase C and mitogen-activated protein kinase phosphatase-1 in vascular smooth muscle[J]. American Journal of Physiology-Cell Physiology, 2016, 310(11): C921-C930,Trappanese D M, Sivilich S, Ets H K, et al.
· ModProt: a database for integrating laboratory and literature data about protein post-translational modifications[J]. Journal of Electrophoresis, 2016, 60(1): 1-4,Kimura Y, Toda T, Hirano H.
· The C-ETS2-TFEB Axis Promotes Neuron Survival under Oxidative Stress by Regulating Lysosome Activity[J]. Oxidative medicine and cellular longevity, 2016,Ma S, Fang Z, Luo W, et al.
· Essential role of the PSI–LHCII supercomplex in photosystem acclimation to light and/or heat conditions by state transitions[J]. Photosynthesis Research, 2016: 1-10,Marutani Y, Yamauchi Y, Higashiyama M, et al.
· Identification of a redox-modulatory interaction between selenoprotein W and 14-3-3 protein[J]. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2016, 1863(1): 10-18,Jeon Y H, Ko K Y, Lee J H, et al.
· Effects of hydrogen sulfide on the heme coordination structure and catalytic activity of the globin-coupled oxygen sensor AfGcHK[J]. BioMetals, 2016, 29(4): 715-729,Fojtikova V, Bartosova M, Man P, et al.
· Identification and functional analysis of phosphorylation in Newcastle disease virus phosphoprotein[J]. Archives of virology, 2016: 1-14,Qiu X, Zhan Y, Meng C, et al.
· Increased level of phosphorylated desmin and its degradation products in heart failure[J]. Biochemistry and Biophysics Reports, 2016, 6: 54-62,Bouvet M, Dubois-Deruy E, Alayi T D, et al.
· Profiling DNA damage-induced phosphorylation in budding yeast reveals diverse signaling networks[J]. Proceedings of the National Academy of Sciences, 2016: 201602827,Zhou C, Elia A E H, Naylor M L, et al.
· Unexpected properties of sRNA promoters allow feedback control via regulation of a two-component system[J]. Nucleic Acids Research, 2016: gkw642,Brosse A, Korobeinikova A, Gottesman S, et al.
· Evolution of ZnII–Macrocyclic Polyamines to Biological Probes and Supramolecular Assembly[J]. Macrocyclic and Supramolecular Chemistry: How Izatt-Christensen Award Winners Shaped the Field, 2016: 415,Kimura E, Koike T, Aoki S.
· Phosphopeptide Enrichment Using Various Magnetic Nanocomposites: An Overview[J]. Phospho-Proteomics: Methods and Protocols, 2016: 193-209,Batalha Í L, Roque A C A.
· In vivo phosphorylation of a peptide tag for protein purification[J]. Biotechnology letters, 2016, 38(5): 767-772,Goux M, Fateh A, Defontaine A, et al.
· Regulation of cell reversal frequency in Myxococcus xanthus requires the balanced activity of CheY‐like domains in FrzE and FrzZ[J]. Molecular microbiology, 2016,Kaimer C, Zusman D R.
· Elevation of cortical serotonin transporter activity upon peripheral immune challenge is regulated independently of p38 mitogen‐activated protein kinase activation and transporter phosphorylation[J]. Journal of neurochemistry, 2016, 137(3): 423-435,Schwamborn R, Brown E, Haase J.
· The Yeast Cyclin-Dependent Kinase Routes Carbon Fluxes to Fuel Cell Cycle Progression[J]. Molecular cell, 2016, 62(4): 532-545,Ewald J C, Kuehne A, Zamboni N, et al.
· Two Degradation Pathways of the p35 Cdk5 (Cyclin-dependent Kinase) Activation Subunit, Dependent and Independent of Ubiquitination[J]. Journal of Biological Chemistry, 2016, 291(9): 4649-4657,Takasugi T, Minegishi S, Asada A, et al.
· Increased level of phosphorylated desmin and its degradation products in heart failure[J]. Biochemistry and Biophysics Reports. 2016,Bouvet M, Dubois-Deruy E, Alayi T D, et al.
· a high‐affinity LCO‐binding protein of Medicago truncatula, interacts with LYK3, a key symbiotic receptor[J]. FEBS letters, 2016, 590(10): 1477-1487,Fliegmann J, Jauneau A, Pichereaux C, et al. LYR3,
· Nek1 Regulates Rad54 to Orchestrate Homologous Recombination and Replication Fork Stability[J]. Molecular Cell, 2016,Spies J, Waizenegger A, Barton O, et al.
· PhostagTM-gel retardation and in situ thylakoid kinase assay for determination of chloroplast protein phosphorylation targets[J]. Endocytobiosis and Cell Research, 2016, 27(2): 62-70,Dytyuk Y, Flügge F, Czarnecki O, et al.
· Luteinizing Hormone Causes Phosphorylation and Activation of the cGMP Phosphodiesterase PDE5 in Rat Ovarian Follicles, Contributing, Together with PDE1 Activity, to the Resumption of Meiosis[J]. Biology of reproduction, 2016: biolreprod. 115.135897,Egbert J R, Uliasz T F, Shuhaibar L C, et al.
· Newby, AC, & Bond, M.(2016). The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP[J]. Journal of Molecular and Cellular Cardiology, 2016, 90: 1-10,Kimura-Wozniak T, Duggirala A, Smith M C, et al. G.
· Yeast lacking the amphiphysin family protein Rvs167 is sensitive to disruptions in sphingolipid levels[J]. The FEBS Journal, 2016, 283(15): 2911-2928,Toume M, Tani M.
· Regulation of CsrB/C sRNA decay by EIIAGlc of the phosphoenolpyruvate: carbohydrate phosphotransferase system[J]. Molecular microbiology, 2016, 99(4): 627-639,Leng Y, Vakulskas C A, Zere T R, et al.
· The Late S-Phase Transcription Factor Hcm1 Is Regulated through Phosphorylation by the Cell Wall Integrity Checkpoint[J]. Molecular and cellular biology, 2016: MCB. 00952-15,Negishi T, Veis J, Hollenstein D, et al.
· Validation of chemical compound library screening for transcriptional co‐activator with PDZ‐binding motif inhibitors using GFP‐fused transcriptional co‐activator with PDZ‐binding motif[J]. Cancer science, 2016, 107(6): 791-802,Nagashima S, Maruyama J, Kawano S, et al.
· ULK1/2 Constitute a Bifurcate Node Controlling Glucose Metabolic Fluxes in Addition to Autophagy[J]. Molecular cell, 2016, 62(3): 359-370,Li T Y, Sun Y, Liang Y, et al.
· Spatiotemporal dynamics of Oct4 protein localization during preimplantation development in mice[J]. Reproduction, 2016: REP-16-0277,Fukuda A, Mitani A, Miyashita T, et al.
· The tandemly repeated NTPase (NTPDase) from Neospora caninum is a canonical dense granule protein whose RNA expression, protein secretion and phosphorylation coincides with the tachyzoite egress[J]. Parasites & Vectors, 2016, 9(1): 1,Pastor-Fernández I, Regidor-Cerrillo J, Álvarez-García G, et al.
· Interaction Analysis of a Two-Component System Using Nanodiscs[J]. PloS one, 2016, 11(2): e0149187,Hörnschemeyer P, Liss V, Heermann R, et al.
· Constitutive Activation of PINK1 Protein Leads to Proteasome-mediated and Non-apoptotic Cell Death Independently of Mitochondrial Autophagy[J]. Journal of Biological Chemistry, 2016, 291(31): 16162-16174,Akabane S, Matsuzaki K, Yamashita S, et al.
· p38β Mitogen-Activated Protein Kinase Modulates Its Own Basal Activity by Autophosphorylation of the Activating Residue Thr180 and the Inhibitory Residues Thr241 and Ser261[J]. Molecular and cellular biology, 2016, 36(10): 1540-1554,Beenstock J, Melamed D, Mooshayef N, et al.
· Lysophosphatidylcholine acyltransferase 1 protects against cytotoxicity induced by polyunsaturated fatty acids[J]. The FASEB Journal, 2016, 30(5): 2027-2039,Akagi S, Kono N, Ariyama H, et al.
· Characterization of a herpes simplex virus 1 (HSV-1) chimera in which the Us3 protein kinase gene is replaced with the HSV-2 Us3 gene[J]. Journal of virology, 2016, 90(1): 457-473,Shindo K, Kato A, Koyanagi N, et al.
· Generation of phospho‐ubiquitin variants by orthogonal translation reveals codon skipping[J]. FEBS letters, 2016, 590(10): 1530-1542,George S, Aguirre J D, Spratt D E, et al.
· Evolution of KaiC-Dependent Timekeepers: A Proto-circadian Timing Mechanism Confers Adaptive Fitness in the Purple Bacterium Rhodopseudomonas palustris[J]. PLoS Genet, 2016, 12(3): e1005922,Ma P, Mori T, Zhao C, et al.
· Phosphorylation of Bni4 by MAP kinases contributes to septum assembly during yeast cytokinesis[J]. FEMS Yeast Research, 2016, 16(6): fow060,Pérez J, Arcones I, Gómez A, et al.
· Alteration of Antiviral Signalling by Single Nucleotide Polymorphisms (SNPs) of Mitochondrial Antiviral Signalling Protein (MAVS)[J]. PloS one, 2016, 11(3): e0151173,Xing F, Matsumiya T, Hayakari R, et al.
· Arm-in-arm response regulator dimers promote intermolecular signal transduction[J]. Journal of bacteriology, 2016, 198(8): 1218-1229,Baker A W, Satyshur K A, Morales N M, et al.
· The lsh/ddm1 homolog mus-30 is required for genome stability, but not for dna methylation in neurospora crassa[J]. PLoS Genet, 2016, 12(1): e1005790,Basenko E Y, Kamei M, Ji L, et al.
· Fine tuning chloroplast movements through physical interactions between phototropins[J]. Journal of Experimental Botany, 2016: erw265,Sztatelman O, Łabuz J, Hermanowicz P, et al.
· Characterization of the Neospora caninum NcROP40 and NcROP2Fam-1 rhoptry proteins during the tachyzoite lytic cycle[J]. Parasitology, 2016, 143(01): 97-113,Pastor-Fernandez I, Regidor-Cerrillo J, Jimenez-Ruiz E, et al.
· Transcriptional Profile during Deoxycholate-Induced Sporulation in a Clostridium perfringens Isolate Causing Foodborne Illness[J]. Applied and environmental microbiology, 2016, 82(10): 2929-2942,Yasugi M, Okuzaki D, Kuwana R, et al.
· Timely Closure of the Prospore Membrane Requires SPS1 and SPO77 in Saccharomyces cerevisiae[J]. Genetics, 2016: genetics. 115.183939,Paulissen S M, Slubowski C J, Roesner J M, et al.
· DDK dependent regulation of TOP2A at centromeres revealed by a chemical genetics approach[J]. Nucleic Acids Research, 2016: gkw626,Wu K Z L, Wang G N, Fitzgerald J, et al.
· OVATE Family Protein 8 Positively Mediates Brassinosteroid Signaling through Interacting with the GSK3-like Kinase in Rice[J]. PLoS Genet, 2016, 12(6): e1006118,Yang C, Shen W, He Y, et al.
· Epithelial Sel1L is required for the maintenance of intestinal homeostasis[J]. Molecular biology of the cell, 2016, 27(3): 483-490, Sun S, Lourie R, Cohen S B, et al.
· Effect of Sodium Dodecyl Sulfate Concentration on Supramolecular Gel Electrophoresis[J]. ChemNanoMat, 2016,Tazawa S, Kobayashi K, Yamanaka M.
· Intergenic VNTR Polymorphism Upstream of rocA Alters Toxin Production and Enhances Virulence in Streptococcus pyogenes[J]. Infection and immunity, 2016: IAI. 00258-16,Zhu L, Olsen R J, Horstmann N, et al.
· Ajuba Phosphorylation by CDK1 Promotes Cell Proliferation and Tumorigenesis[J]. Journal of Biological Chemistry, 2016: jbc. M116. 722751,Chen X, Stauffer S, Chen Y, et al.
· Editorial: International Plant Proteomics Organization (INPPO) World Congress 2014[J]. Frontiers in Plant Science, 2016, 7,Heazlewood J L, Jorrín-Novo J V, Agrawal G K, et al.
· Phosphoinositide kinase signaling controls ER-PM cross-talk[J]. Molecular biology of the cell, 2016, 27(7): 1170-1180,Omnus D J, Manford A G, Bader J M, et al.
· A multiple covalent crosslinked soft hydrogel for bioseparation[J]. Chemical Communications, 2016, 52(15): 3247-3250,Liu Z, Fan L, Xiao H, et al.
· Advances in crop proteomics: PTMs of proteins under abiotic stress[J]. Proteomics, 2016, 16(5): 847-865,Wu X, Gong F, Cao D, et al.
· Cyclin-Dependent Kinase Co-Ordinates Carbohydrate Metabolism and Cell Cycle in S. cerevisiae[J]. Molecular cell, 2016, 62(4): 546-557,Zhao G, Chen Y, Carey L, et al.
· Carbon Monoxide Gas Is Not Inert, but Global, in Its Consequences for Bacterial Gene Expression, Iron Acquisition, and Antibiotic Resistance[J]. Antioxidants & redox signaling, 2016,Wareham L K, Begg R, Jesse H E, et al.
· Two-layer regulation of PAQR3 on ATG14-linked class III PtdIns3K activation upon glucose starvation[J]. Autophagy, 2016: 1-2,Xu D, Wang Z, Chen Y.
· Regulation of sphingolipid biosynthesis by the morphogenesis checkpoint kinase Swe1[J]. Journal of Biological Chemistry, 2016, 291(5): 2524-2534,Chauhan N, Han G, Somashekarappa N, et al.
· PAX5 tyrosine phosphorylation by SYK co-operatively functions with its serine phosphorylation to cancel the PAX5-dependent repression of BLIMP1: A mechanism for antigen-triggered plasma cell differentiation[J]. Biochemical and biophysical research communications, 2016, 475(2): 176-181,Inagaki Y, Hayakawa F, Hirano D, et al.
· A Combined Computational and Genetic Approach Uncovers Network Interactions of the Cyanobacterial Circadian Clock[J]. Journal of Bacteriology, 2016: JB. 00235-16,Boyd J S, Cheng R R, Paddock M L, et al.
· HuR mediates motility of human bone marrow-derived mesenchymal stem cells triggered by sphingosine 1-phosphate in liver fibrosis[J]. Journal of Molecular Medicine, 2016: 1-14,Chang N, Ge J, Xiu L, et al.
· Combined replacement effects of human modified β-hexosaminidase B and GM2 activator protein on GM2 gangliosidoses fibroblasts[J]. Biochemistry and Biophysics Reports, 2016,Kitakaze K, Tasaki C, Tajima Y, et al.
· Roseotoxin B Improves Allergic Contact Dermatitis through a Unique Anti-inflammatory Mechanism Involving Excessive Activation of Autophagy in Activated T-Lymphocytes[J]. Journal of Investigative Dermatology, 2016,Wang X, Hu C, Wu X, et al.
References on Phos-tag™ Chemistry
-
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of phosphorylated compounds using a novel phosphate capture molecule, Rapid Communications of Mass Spectrometry, 17, 2075-2081 (2003), H. Takeda, A. Kawasaki, M. Takahashi, A. Yamada, and T. Koike
-
Recognition of phosphate monoester dianion by an alkoxide-bridged dinuclear zinc (II) complex, Dalton Transactions, 1189-1193 (2004), E. Kinoshita, M. Takahashi, H. Takeda, M. Shiro, and T. Koike
-
Quantitative analysis of lysophosphatidic acid by time-of-flight mass spectrometry using a phosphate capture molecule, Journal of Lipid Research, 45, 2145-2150 (2004), T. Tanaka, H. Tsutsui, K. Hirano, T. Koike, A. Tokumura, and K. Satouchi
-
Production of 1,2-Didocosahexaenoyl Phosphatidylcholine by Bonito Muscle Lysophosphatidylcholine/Transacylase, Journal of Biochemistry,136, 477-483 (2004), K. Hirano, H. Matsui, T. Tanaka, F. Matsuura, K. Satouchi, and T. Koike
-
Novel immobilized zinc(II) affinity chromatography for phosphopeptides and phosphorylated proteins, Journal of Separation Science, 28, 155-162 (2005), E. Kinoshita, A. Yamada, H. Takeda, E. Kinoshita-Kikuta, and T. Koike
-
Detection and Quantification of On-Chip Phosphorylated Peptides by Surface Plasmon Resonance Imaging Techniques Using a Phosphate Capture Molecule, Analytical Chemistry, 77, 3979-3985 (2005), K. Inamori, M. Kyo, Y. Nishiya, Y. Inoue, T. Sonoda, E. Kinoshita, T. Koike, and Y. Katayama
-
Phosphate-binding tag: A new tool to visualize phosphorylated proteins, Molecular & Cellular Proteomics, 5, 749-757 (2006), E. Kinoshita, E. Kinoshita-Kikuta, K. Takiyama, and T. Koike
-
Enrichment of phosphorylated proteins from cell lysate using phosphate-affinity chromatography at physiological pH, Proteomics, 6, 5088-5095 (2006), E. Kinoshita-Kikuta, E. Kinoshita, A. Yamada, M. Endo, and T. Koike
-
Separation of a phosphorylated histidine protein using phosphate affinity polyacrylamide gel electrophoresis, Analytical Biochemistry, 360, 160-162 (2007), S. Yamada, H. Nakamura, E. Kinoshita, E. Kinoshita-Kikuta, T. Koike, and Y. Shiro
-
Label-free kinase profiling using phosphate-affinity polyacrylamide gel electrophresis, Molecular & Cellular Proteomics, 6, 356-366 (2007), E. Kinoshita-Kikuta, Y. Aoki, E. Kinoshita, and T. Koike
-
A SNP genotyping method using phosphate-affinity polyacrylamide gel electrophoresis, Analytical Biochemistry, 361, 294-298 (2007), E. Kinoshita, E. Kinoshita-Kikuta, and T. Koike (The phosphate group at DNA-terminal is efficiently captured by Zn2+.Phos-tag.)
-
Identification on Membrane and Characterization of Phosphoproteins Using an Alkoxide-Bridged Dinuclear Metal Complex as a Phosphate-Binding Tag Molecule, Journal of Biomolecular Techniques, 18, 278-286 (2007), T. Nakanishi, E. Ando, M. Furuta, E. Kinoshita, E. Kikuta-Kinoshita, T. Koike, S. Tsunasawa, and O. Nishimura
-
A mobility shift detection method for DNA methylation analysis using phosphate affinity polyacrylamide gel electrophoresis, Analytical Biochemistry, 378, 102-104 (2008), E. Kinoshita-Kikuta, E. Kinoshita, and T. Koike
-
Separation of phosphoprotein isotypes having the same number of phosphate groups using phosphate- affinity SDS-PAGE, Proteomics, 8, 2994-3003 (2008), E. Kinoshita, E. Kinoshita-Kikuta, M. Matsubara, S. Yamada, H. Nakamura, Y. Shiro, Y. Aoki, K. Okita, and T. Koike
-
FANCI phosphorylation functions as a molecular switch to turn on the Fanconi anemia pathway, Nature Structural & Molecular Biology, 15, 1138-1146 (2008), M. Ishiai, H. Kitao, A. Smogorzewska, J. Tomida, A. Kinomura, E. Uchida, A. Saberi, E. Kinoshita, E. Kinoshita-Kikuta, T. Koike, S. Tashiro, S. J. Elledge, and M. Takata
-
Two-dimensional phosphate affinity gel electrophoresis for the analysis of phosphoprotein isotypes , Electrophoresis, 30, 550-559 (2009), E. Kinoshita, E. Kinoshita-Kikuta, M. Matsubara, Y. Aoki, S. Ohie, Y. Mouri, and T. Koike
-
Formation of lysophosphatidic acid, a wound-healing lipid, during digestion of cabbage leaves, Bioscience, Biotechnology, and Biochemistry,73, 1293-300 (2009), T. Tanaka, G. Horiuchi, M. Matsuoka, K. Hirano, A. Tokumura, T. Koike, and K. Satouchi
-
A Phos-tag-based fluorescence resonance energy transfer system for the analysis of the dephosphorylation of phosphopeptides, Analytical Biochemistry, 388, 235-241, (2009), K. Takiyama, E. Kinoshita, E. Kinoshita-Kikuta, Y. Fujioka, Y. Kubo, and T. Koike
-
Phos-tag beads as an immunoblotting enhancer for selective detection of phosphoproteins in cell lysates, Analytical Biochemistry, 389, 83-85, (2009), E. Kinoshita-Kikuta, E. Kinoshita, and T. Koike
-
Mobility shift detection of phosphorylation on large proteins using a Phos-tag SDS-PAGE gel strengthened with agarose, Proteomics, 9, 4098- 4101 (2009), E. Kinoshita, E. Kinoshita-Kikuta, H. Ujihara, and T. Koike
-
Separation and detection of large phosphoproteins using Phos-tag SDS-PAGE, Nature Protocols, 4, 1513-1521 (2009), E. Kinoshita, E. Kinoshita-Kikuta, and T. Koike
-
A clean-up technology for the simultaneous determination of lysophosphatidic acid and sphingosine-1-phosphate by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using a phosphate-capture molecule, Phos-tag, Rapid Communications in Mass Spectrometry, 24, 1075-1084 (2010), J. Morishige, M. Urikura, H. Takagi, K. Hirano, T. Koike, T. Tanaka, and K. Satouchi
-
Genotyping and mapping assay of single-nucleotide polymorphisms in CYP3A5 using DNA-binding zinc(II) complexes, Clinical Biochemistry, 43, 302-306 (2010), E. Kinoshita, E. Kinoshita-Kikuta, H. Nakashima, and T. Koike
-
The DNA-binding activity of mouse DNA methyltransferase 1 is ragulated phosphorylation with casein kinase 1σ/ε, Biochemical Journal, 427, 489-497 (2010), Y. Sugiyama, N. Hatano, N. Sueyoshi, I. Suetake, S. Tajima, E. Kinoshita, E. Kinoshita-Kikuta, T. Koike, and I. Kameshita
to Page top
